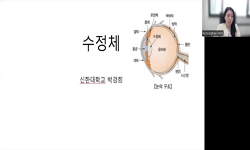
The elution profile of nonmethylated and endogenous methylated soluble fraction protein of bovine lens are compared by sephacryl S-300 chromatography, and then changes of protein patterns of nonmethylated and methylated α-crystallin fractions have be...
http://chineseinput.net/에서 pinyin(병음)방식으로 중국어를 변환할 수 있습니다.
변환된 중국어를 복사하여 사용하시면 됩니다.
- 中文 을 입력하시려면 zhongwen을 입력하시고 space를누르시면됩니다.
- 北京 을 입력하시려면 beijing을 입력하시고 space를 누르시면 됩니다.
https://www.riss.kr/link?id=T170388
- 저자
-
발행사항
대전 : 忠南大學校 大學院, 1985
- 학위논문사항
-
발행연도
1985
-
작성언어
한국어
- 주제어
-
KDC
515.76 판사항(3)
-
발행국(도시)
대전
-
형태사항
6 p. ; 26 cm .
- 소장기관
-
0
상세조회 -
0
다운로드
부가정보
다국어 초록 (Multilingual Abstract)
1. The nonmethylated and methylated soluble fraction proteins are analyzed by sephacryl S-300 chromatography. In the nonmethylated protein four peaks are eluted in the tube NO. 21, 30, 34, 38 and identified as α, β_H,β_L,γ-crystallin, but eluted in the tube NO. 20, 29, 32, 36 in methylated protein, respectively.
2. When the soluble fraction of lens is methylated with S-adenosyl-L- [methyl-³H] methionine and separated by sephacryl S-300 chromatography, 90% of radioactivity is detected in α-crystallin fraction.
3. Nonmethylated α-crystallin is separated eight subfractions by urea/polyacrylamide gel electrophoresis. In the methylated α-crystallin, subfraction NO. 6,7 are disappeared and NO. 4, 5 are increased when compared with that of nonmethylated.
From the above result, it is suggested that endogenous methylation of α-crystallin of lens by protein methylase Ⅱ may be related to the formation of high molecular weight protein.
The elution profile of nonmethylated and endogenous methylated soluble fraction protein of bovine lens are compared by sephacryl S-300 chromatography, and then changes of protein patterns of nonmethylated and methylated α-crystallin fractions have been investigated by urea/ polyacrylamide gel electrophoresis.
1. The nonmethylated and methylated soluble fraction proteins are analyzed by sephacryl S-300 chromatography. In the nonmethylated protein four peaks are eluted in the tube NO. 21, 30, 34, 38 and identified as α, β_H,β_L,γ-crystallin, but eluted in the tube NO. 20, 29, 32, 36 in methylated protein, respectively.
2. When the soluble fraction of lens is methylated with S-adenosyl-L- [methyl-³H] methionine and separated by sephacryl S-300 chromatography, 90% of radioactivity is detected in α-crystallin fraction.
3. Nonmethylated α-crystallin is separated eight subfractions by urea/polyacrylamide gel electrophoresis. In the methylated α-crystallin, subfraction NO. 6,7 are disappeared and NO. 4, 5 are increased when compared with that of nonmethylated.
From the above result, it is suggested that endogenous methylation of α-crystallin of lens by protein methylase Ⅱ may be related to the formation of high molecular weight protein.
목차 (Table of Contents)
- 목차
- I. 緖論 = 1
- II. 實驗材料 및 方法 = 1
- III. 實驗成績 = 2
- IV. 考察 = 4
- 목차
- I. 緖論 = 1
- II. 實驗材料 및 方法 = 1
- III. 實驗成績 = 2
- IV. 考察 = 4
- V. 結論 = 4
- 參考文獻 = 5
- Summary = 6